Arrhenius Number

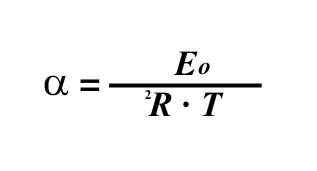
The Arrhenius number (Ar) is a dimensionless parameter used in heat transfer analysis to characterize the relative importance of thermal diffusion to fluid advection in a fluid flow situation. It’s named after the Swedish chemist Svante Arrhenius.
Arrhenius number is proportional to { (activation energy) / (potential energy) } and is used in mass transfer in general and reaction rate calculations in particular. It is normally defined in the following form
Where: | ||
Eo | = | Activation Energy |
R | = | Gas law constant |
T | = | Temperature |
The Arrhenius number helps determine whether thermal diffusion or fluid advection dominates the overall heat transfer process. Depending on the value of the Arrhenius number, different heat transfer regimes can be identified:
- If Ar << 1, thermal diffusion dominates, and heat transfer occurs mainly through conduction. In this regime, temperature gradients are smoothed out relatively quickly compared to fluid flow.
- If Ar >> 1, fluid advection dominates, and heat transfer occurs mainly through convection. In this regime, temperature gradients are advected by the fluid flow, leading to significant temperature variations.
The Arrhenius number is commonly used in the analysis of heat transfer in fluid flows, such as in convective heat transfer in pipes, channels, and boundary layers. It helps engineers and scientists understand and predict the heat transfer behavior of fluids under different flow conditions.

