Enthalpy Unit Conversion (Mass Basis)” refers to the process of converting enthalpy values from one unit of mass to another. Enthalpy is a measure of the total energy of a thermodynamic system and is often expressed per unit mass.
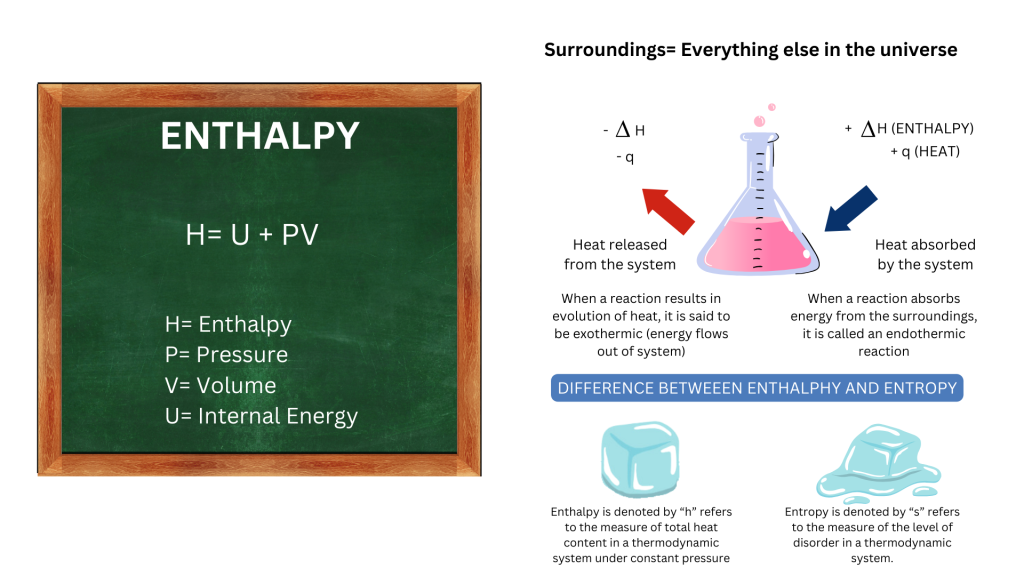
Enthalpy change can be defined simply as the energy used to break existing bonds and the energy released when new bonds form. It considers the energy levels of both the initial substances and the resulting products.
For example, if enthalpy is given in units of kilojoules per kilogram (kJ/kg), and you need to convert it to units of British Thermal Units per pound (BTU/lb), you would perform an enthalpy unit conversion on a mass basis. This conversion ensures consistency and accuracy in expressing enthalpy values across different measurement systems or units.